LDN: What It Is, How It Works, and What It Treats
In 1984 Naltrexone was approved by the FDA in the USA for the treatment of opioid addiction, used at the standard dose of 50mg to 100mg per day. It is a pure antagonist at various opioid receptors, Delta Kappa, Mu, and Opioid Growth Factor (OGF) receptors. LDN is low dose naltrexone, and used at much lower doses than when first used. It reduces pain, and fights inflammation. It is used to treat cancers, autoimmune diseases, chronic pain and mental health issues, to name a few. Treatment is constantly evolving, with new conditions and methods of treatment being shared regularly. LDN is a safe, non-toxic and inexpensive drug that helps regulate a dysfunctional immune system. Low-dose Naltrexone (LDN) has emerged as a significant player in the realm of alternative therapeutics, particularly for chronic conditions that have perplexed the medical community.
Originally developed as an opioid receptor antagonist at higher doses, LDN exhibits a paradoxical effect at lower doses, leading to enhanced endogenous opioid production. This unique mechanism positions it as a potential treatment for a variety of ailments, including autoimmune diseases and pain disorders. Research indicates that LDN may modulate immune responses, benefiting conditions such as fibromyalgia and multiple sclerosis, where inflammation plays a crucial role. LDNs functionality as a TLR4 antagonist and modulator of microglial cell activity contributes to its anti-inflammatory properties, reinforcing its therapeutic promise. As the scientific community continues to explore its benefits, understanding the intricacies of LDNs action and optimising its’ clinical applications, we will improved patient outcomes.
Overview of Low-Dose Naltrexone (LDN) and its significance in modern medicine
Low-Dose Naltrexone (LDN) has emerged as a significant therapeutic agent in modern medicine, particularly for managing chronic conditions such as autoimmune diseases, fibromyalgia, chronic fatigue syndrome, depression, mould disease, CIRS, and as adjunctive therapy for certain forms of cancer. By using a fraction of the standard opioid-blocking dose, LDN operates through mechanisms that enhance endogenous opioid production and modulate immune responses, fostering a more balanced bodily state. The shift towards recognising patient-generated health data has assisted in highlighting potential uses of LDN.
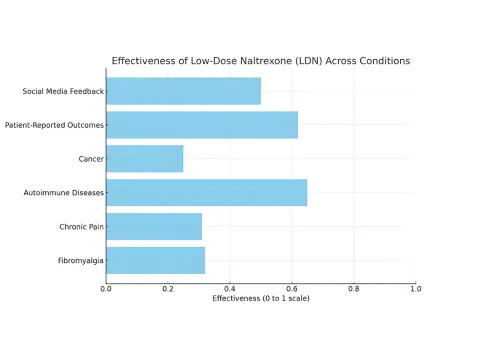
The chart displays the effectiveness of Low-Dose Naltrexone (LDN) across various medical conditions. It highlights that Autoimmune Diseases and Patient-Reported Outcomes show the highest effectiveness, while Cancer has the lowest. Social Media Feedback also indicates a notable impact, suggesting diverse perspectives on LDN's effectiveness among patients.
Understanding LDN
To appreciate the therapeutic potential of Low Dose Naltrexone (LDN), it is essential to understand its pleiotropic mechanisms and clinical applications. Primarily, LDN acts as an antagonist at opioid receptors, which aids in modulating immune responses and reducing inflammation. This is particularly beneficial for conditions such as autoimmune diseases and chronic pain syndromes. Notably, LDNs stimulating effects on endogenous opioid production contribute to its analgesic properties, making it effective in managing symptoms of fibromyalgia and multiple sclerosis.
There are intricate signalling pathways involved, as depicted in Figure 1 below, which depict its potential mechanisms of action in reducing inflammation and modulating immune response.. Understanding the influence of genetic variants on LDNs efficacy can also enhance personalised medicine approaches, allowing for tailored therapeutic strategies that address individual patient needs (Lent JV et al., p. 1348-1364)(Green M et al., p. 43-57). Consequently, ongoing research is crucial for elucidating LDNs full potential as a treatment modality.
Definition and chemical composition of Low-Dose Naltrexone
Low-dose naltrexone (LDN) is a pharmacological agent derived from naltrexone, an opioid receptor antagonist originally used to treat opioid dependence. Defined as a low-dose variant ranging from 1 to 5 mg, LDN operates on the principle of temporarily blocking opioid receptors, which in turn promotes an upregulation of endorphins—natural pain-relieving compounds produced by the body. Its chemical composition retains the same core structure as naltrexone but exhibits distinct pharmacokinetic properties due to the significantly reduced dosage.
This unique mechanism positions LDN as a potential therapeutic option for various autoimmune diseases and chronic pain conditions, leveraging its capacity to modulate the immune response and reduce inflammation. The importance of understanding LDN’s biochemical interactions and its’ role in TLR4 receptor antagonism and cytokine regulation, allows clinical applications to be explored in conditions like Long COVID and autoimmune disorders
Mechanism of Action
Low-dose Naltrexone (LDN) operates through a multifaceted mechanism that primarily involves modulation of opioid receptors as well as inhibition of pro-inflammatory signalling pathways. Understanding how LDN works requires a grasp of three fundamental biological principles. First, opiate receptors are present in multiple biological systems in the human body, as they regulate a great number of biological functions via the central release of natural opiates (endorphins/met-enkephalins).
Second, a class of proteins called toll-like receptors (TLRs) are part of the immune system, providing a first line of defence against microbial invasion and possessing the ability to recognise and be activated by not only pathogens but also endogenous signalling molecules. Lastly, naltrexone, when given at a low dose, has antagonistic activity in both of these areas, and is able to modify biological functions of these receptor groups by suppressing unwanted immune reactions, or by stimulating disease-suppressed immune activity.
At low doses, LDN temporarily blocks opioid receptors, prompting a rebound effect that leads to increased production of endorphins and enkephalins, which are vital for pain relief and immune modulation. This unique mechanism contrasts sharply with the effects observed at higher doses, where sustained opioid blockade occurs, underscoring the concept of hormesis. Furthermore, LDN acts as an antagonist to the TLR4 receptor, reducing microglial cell activation and pro-inflammatory cytokine release, which has been shown to play a crucial role in neuroinflammatory conditions. Controlling inflammation is critical for most health conditions. Inflammation can cause a dysfunctional immune system, causing a shift from a heathy T1 dominant system to an unhealthy TH2 / TH17 dominant system.
This shift, dominated by worsening inflammation and autoimmunity contributes to mitochondrial dysfunction, reduced ability to fight intracellular infections, impairment of the conversion of IgM to IgG, thymic dysfunction, T cell exhaustion, cellular and immunosenecence, excessive mast cell activation, immune activation of coagulation, reduced detox capacity, and low adrenal, thyroid and growth hormone levels. Understanding LDN’s diverse actions, including those related to neuroinflammation, provides insight into its therapeutic potential for various conditions, including autoimmune diseases and chronic pain syndromes ((Androphy et al.)).
How LDN interacts with the body’s opioid receptors and immune system
Low-dose naltrexone (LDN) uniquely interacts with the body’s opioid receptors and immune system, providing a multifaceted therapeutic approach. Primarily, LDN operates as an antagonist at opioid receptors; however, its low dosage leads to a paradoxical effect—upregulating natural endorphin production after the blockade wears off. This dynamic has significant implications for chronic pain management and inflammatory conditions, as the body compensates by enhancing its endogenous opioid activity. Additionally, LDN influences the immune response by modulating the activity of Toll-like receptor 4 (TLR4), which is essential in the body’s inflammatory processes. Such immune modulation aids in reducing pro-inflammatory cytokine release, thereby improving conditions characterised by overactive immune responses, such as autoimmune diseases (Metz et al.).
Naltrexone exists in a racemic mixture of isomers ("left-handedness and right-handedness")
• Dextro-naltrexone (right) binds toll-like receptors (TLR)
• Levo-naltrexone (left) binds opioid receptors
Dextro-naltrexone
• Antagonist effect at Toll-like receptors (TLR)
• TLR-4 receptors exist on microglial cells, other macrophages, mast cells
• Activated microglial cells produce proinflammatory cytokines, substance P, nitric oxide
• Inhibition leads to a decreased proinflammatory cascade
Levo-naltrexone
• Antagonist effect at opioid receptors
• Small temporary opioid blockade
• Upregulates endogenous opioid production
• Upregulates opioid receptors
• Increased endorphins favourable to the immune system
The interplay between opioid receptors and immune responses presents a novel pathway for LDN’s effectiveness, meriting further exploration in therapeutic settings (Sonis D et al.). To visualise this interaction, effectively illustrates the complex signalling pathways involved, highlighting its importance in the broader context of pain management and immune regulation.
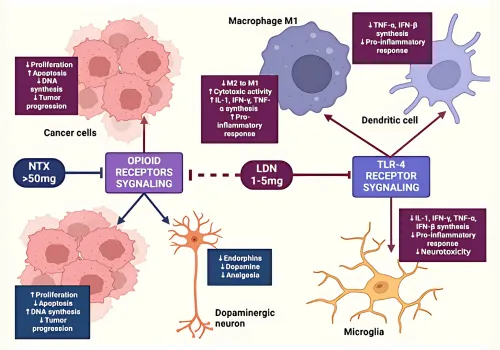
Image1. Cell Signaling Pathways Involving Opioid Receptors and TLR-4 in Cancer and Neuroinflammation
Therapeutic Applications
The therapeutic applications of Low-Dose Naltrexone (LDN) are becoming increasingly recognised, particularly for its role in managing diverse conditions such as fibromyalgia, chronic fatigue, multiple sclerosis, depression, auto-immune diseases such as Hashimoto’s thyroiditis, viral damaged tissue, cancer, mould disease, CIRS and inflammatory bowel diseases like Crohns disease.
These ailments commonly present complex pathophysiological challenges that traditional treatments often fail to address adequately. LDN operates through complex mechanisms, including antagonising Toll-Like Receptor 4 (TLR4) signalling, which may help mitigate inflammation and promote healing in various cell types, including macrophages and microglia . Furthermore, LDNs modulation of opioid receptor activity enhances the body’s endogenous pain control systems, making it a promising candidate for pain relief and improved quality of life in affected patients. Emerging research also highlights its potential effectiveness in cancer therapies by influencing tumour behaviour and immune responses, expanding its effectiveness across therapeutic contexts (Androphy et al.)(Weßeler et al.).
Study
Condition
Sample Size
LDN Dose
Duration
Outcome
Source
Retrospective Cohort Study on Chronic Pain
Chronic Pain
78 patients (36 LDN users, 42 controls)
Not specified
≥2 months
LDN users reported a 37.8% pain reduction compared to 4.3% in controls (p < 0.001). Multivariate modeling predicted a 33% pain reduction with LDN, with a number needed to treat of 3.2 for 50% pain reduction.
https:// pubmed.ncbi. nlm.nih.gov/ 35289682/
Retrospective Observationa l Cohort Study on Chronic Pain
Chronic Pain
31 patients
≤10 mg
≥1 month
Mean Pain, Enjoyment of Life, and General Activity (PEG) scores improved from 7.27 to 6.62 (mean difference 0.66, 95% CI [0.10-1.21], p = 0.022). 87% discontinued LDN, with 52% citing lack of benefit.
https:// pubmed.ncbi. nlm.nih.gov/ 38301136/
Case Series on Chronic Pain
Chronic Pain
70 patients
Not specified
Not specified
64% experienced some relief. Neuropathic pain and complex regional pain syndrome accounted for 51% of responders. Patients with spondylosis were less likely to respond to LDN (p = 0.00435).
https:// pubmed.ncbi. nlm.nih.gov/ 37337611/
Systematic Review on Fibromyalgia
Fibromyalgia
Not specified
Not specified
Not specified
LDN was found effective in symptomatic management of fibromyalgia. No severe adverse events were reported in 78% of studies evaluating safety.
https:// pubmed.ncbi. nlm.nih.gov/ 36974308/
Pilot Study on Arthritis Pain
Osteoarthritis and Inflammatory Arthritis
Not specified
4.5 mg
8 weeks
Primary outcome: differences in pain interference during LDN and placebo periods. Secondary outcomes: changes in mean pain severity, fatigue, depression, and healthrelated quality of life
https:// pubmed.ncbi. nlm.nih.gov/ 37045708/
Conditions treated by LDN, including autoimmune diseases and chronic pain
Low-dose naltrexone (LDN) is increasingly recognised for its therapeutic potential in treating a range of conditions, particularly autoimmune diseases and chronic pain disorders. Conditions such as Crohn’s disease and fibromyalgia have shown promise in preliminary studies, suggesting that LDN may reduce pain symptoms and improve overall quality of life without adverse effects (Tkach et al.). Additionally, recent evidence highlights its efficacy in managing pruritus associated with systemic sclerosis, emphasizing LDN’s role as a highly tolerable treatment option for skin and gastrointestinal symptoms (Frech et al.).
LDN may also pal role in anti-aging protocol as it does appear to up regulate a unique gene expression affecting autophagy, and also seems to increase the activity of pro-apoptotic genes, BAD and BIK1. As interest in alternative therapies grows amidst the opioid crisis, LDN presents a compelling alternative due to its mechanism, which appears to modulate immune responses and enhance endogenous analgesia . With a high safety profile and increasing anecdotal and clinical support, further research is needed to solidify LDNs place in the treatment protocol for these challenging conditions, paving the way for larger, randomised controlled trials.
Conclusion
In conclusion, the exploration of Low-dose Naltrexone (LDN) reveals its potential as a versatile therapeutic option across various medical conditions, particularly in managing chronic pain, auto-immune and inflammatory diseases. Through its unique mechanisms of action, including TLR4 antagonism and modulation of endogenous opioid signalling, LDN has shown promise in treating ailments such as fibromyalgia and multiple sclerosis, among others. Although further research is essential to confirm its efficacy and optimal dosing regimens, current studies point to LDNs ability to enhance patient quality of life by reducing symptoms associated with these challenging conditions. The intricate interplay of LDN with genetic and metabolic factors, as illustrated above and supported by findings in (Androphy et al.) and (Cooper et al.), underscores the importance of a personalised approach in its application.
As interest in repurposing existing medications continues to grow, LDN stands at the forefront of innovative treatment strategies. LDN has very little potential drug interaction issues (apart from narcotic drugs), and may be used in integrative polypharmacy protocols with agents such as: zinc, NAD+, SPM, quercetin, resveratrol, and NAC.
Summary of LDN's potential benefits and future directions in research
The exploration of Low-dose Naltrexone (LDN) continues to unveil its potential benefits, particularly in managing various chronic conditions such as fibromyalgia, CFS, multiple sclerosis, auto-immune conditions, mould illness, CIRS, IBD, IBS and cancer. Emerging research suggests LDN may alleviate pain and improve quality of life through its mechanisms involving opioid growth factor antagonism and modulation of the central nervous systems inflammatory responses, as indicated in studies focusing on chronic pain management (Borràs et al.).
Future research directions, particularly randomised controlled trials with long-term follow-ups, promise to refine our understanding of LDNs efficacy and safety in diverse populations. Ongoing investigations into genetic factors influencing LDN metabolism and efficacy could provide personalised treatment options, significantly enhancing patient outcomes (Forsberg H et al.). LDN can, and will continue to provide further relief for significant human suffering.
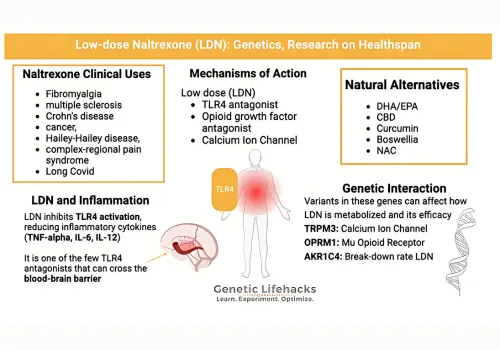
Image2. Overview of Low-dose Naltrexone and its Clinical Applications
Choose the level of care that matches your goals.
Each tier offers personalized support, science-backed protocols, and exclusive tools to help you thrive at every stage of your longevity journey.
Longevity Health Assessment Program
(Initial consult + 14 Day Digital Eat Green Challenge $399)
Healthy Hormones Program (Initial consult + 60 day digital Healthy Hormones program $450)
10 Week Weight Loss Program (Initial consult + 10 week digital weight loss program $299)
Peptides Research Program (Initial consult + 60 Day digital Health & Wellbeing Plan $399)

LDN Program
(Initial consult + 60 Day Health & Wellbeing Plan $399)
Other recommended tests (extra fees apply)
Heart rate variability assessment.
Autonomic nervous system assessment.
Physical / mental stress assessment.
Chronic fatigue & electro-cardiac stability assessment.
Blood vessel ageing and arterial elasticity assessment.
RSA (respiratory sinus arrhythmia training session to recover autonomic balance and vagal tone).
Resting metabolic rate analysis.
Dexa Body composition and bone density analysis.
Olfactory test for Parkinson’s and Alzheimers disease.
Grip strength assessment.
VO2 Max aerobic capacity analysis.
CT coronary angiography.
Book A Consultation
Initial Integrative Consult
45mins - 1 hr | $245 - $400
Integrative Consult Follow-up
20 - 30 mins | $165
Standard
Consult
10 - 15 mins | $95
Healthy Hormones Consult
Initial consult $450
Follow up hormone consults $280

COMPANY
CUSTOMER CARE
FOLLOW US
Copyright 2026. Omics Longevity. All Rights Reserved.